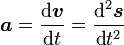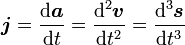Infobox references
Function in biology[edit]
Glucose is the most widely used aldohexose in living organisms. One possible explanation for this is that glucose has a lower tendency than other aldohexoses to react non-specifically with the amine groups of proteins.[4] This reaction –glycation – impairs or destroys the function of many proteins.[4] Glucose’s low rate of glycation can be attributed to it having a more stable cyclic form compared to other aldohexoses, which means it spends less time than they do in its reactive open-chain form.[4] The reason for glucose having the most stable cyclic form of all the aldohexoses is due it having all of its hydroxy groups (with the exception of the hydroxy group on the anomeric carbon of D-glucose) in theequatorial position. Many of the long-term complications of diabetes (e.g., blindness, renal failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids.[5] In contrast, enzyme-regulated addition of sugars to protein is called glycosylation and is essential for the function of many proteins.[6]
Analyte in medical blood test[edit]
Main article: Glucose testGlucose is a common medical analyte measured in blood samples. Eating or fasting prior to taking a blood sample has an effect on the result. A high fasting glucose blood sugar level may be a sign of prediabetes or diabetes mellitus.
Energy source[edit]
Glucose is a ubiquitous fuel in biology. It is used as an energy source in most organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration, or fermentation. Glucose is the human body’s key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoules) of food energy per gram.[7]Breakdown of carbohydrates (e.g. starch) yields mono- and disaccharides, most of which is glucose. Throughglycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form CO2 and water, yielding energy mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood.
Glucose is a primary source of energy for the brain, so its availability influences psychological processes. Whenglucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired.[8][9][10][11]
Glycolysis[edit]

Glucose metabolism and various forms of it in the process
Glucose-containing compounds andisomeric forms are digested and taken up by the body in the intestines, including starch, glycogen,disaccharides and monosaccharides.
Glucose is stored in mainly the liver and muscles as glycogen.
It is distributed and used in tissues as free glucose.
Use of glucose as an energy source in cells is by either aerobic respiration, anaerobic respiration, or fermentation. All of these processes follow from an earlier metabolic pathway known as glycolysis. The first step of glycolysis is thephosphorylation of glucose by a hexokinase to form glucose 6-phosphate. The main reason for the immediate phosphorylation of glucose is to prevent its diffusion out of the cell as the charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane. Furthermore, addition of the high-energy phosphate groupactivates glucose for subsequent breakdown in later steps of glycolysis. At physiological conditions this initial reaction is irreversible.
In anaerobic respiration, one glucose molecule produces a net gain of two ATP molecules (four ATP molecules are produced during glycolysis, but two are required by enzymes used during the process).[12] In aerobic respiration, a molecule of glucose is much more profitable in that a maximum net production of 30 or 32 ATP molecules (depending on the organism) is generated.[13]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
[[File:
|{{{bSize}}}px|alt=Glycolysis and Gluconeogenesis edit]]
Glycolysis and Gluconeogenesis edit
Precursor[edit]
Organisms use glucose as a precursor for the synthesis of several important substances. Starch, cellulose, and glycogen (“animal starch”) are common glucosepolymers (polysaccharides). Some of these polymers (starch or glycogen) serve as energy stores, while others (cellulose and chitin, which is made from a derivative of glucose) have structural roles. Oligosaccharides of glucose combined with other sugars serve as important energy stores. These include lactose, the predominant sugar in milk, which is a glucose-galactose disaccharide, and sucrose, another disaccharide which is composed of glucose and fructose. Glucose is also added onto certain proteins and lipids in a process called glycosylation. This is often critical for their functioning. The enzymes that join glucose to other molecules usually use phosphorylated glucose to power the formation of the new bond by coupling it with the breaking of the glucose-phosphate bond.
Other than its direct use as a monomer, glucose can be broken down to synthesize a wide variety of other biomolecules. This is important, as glucose serves both as a primary store of energy and as a source of organic carbon. Glucose can be broken down and converted into lipids. It is also a precursor for the synthesis of other important molecules such as vitamin C (ascorbic acid).
Structure and nomenclature[edit]
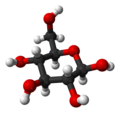
Glucose is a monosaccharide with formula C6H12O6 or H-(C=O)-(CHOH)5-H, whose five hydroxyl (OH) groups are arranged in a specific way along its six-carbonback.
Open-chain form[edit]

Glucose can exist in both a straight-chain and ring form.

D-Glucose in Fischer projection
In its fleeting open-chain form, the glucose molecule has an open (as opposed to cyclic) and unbranched backbone of six carbon atoms, C-1 through C-6; where C-1 is part of an aldehyde group H(C=O)-, and each of the other five carbons bears one hydroxyl group -OH. The remaining bonds of the backbone carbons are satisfied by hydrogen atoms -H. Therefore glucose is both a hexose and an aldose, or analdohexose. The aldehyde group makes glucose a reducing sugar giving a positive reaction with theFehling test.
Each of the four carbons C-2 through C-5 is a stereocenter, meaning that its four bonds connect to four different substituents. (Carbon C-2, for example, connects to -(C=O)H, -OH, -H, and -(CHOH)4H.) In D-glucose, these four parts must be in a specific three-dimensional arrangement. Namely, when the molecule is drawn in the Fischer projection, the hydroxyls on C-2, C-4, and C-5 must be on the right side, while that on C-3 must be on the left side.
The positions of those four hydroxyls are exactly reversed in the Fischer diagram of L-glucose. D– and L-glucose are two of the 16 possible aldohexoses; the other 14 are allose, altrose, mannose, gulose, idose, galactose, and talose, each with two enantiomers, “D-” and “L-“.
-
The aldehyde form of glucose
Cyclic forms[edit]
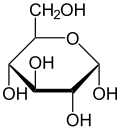
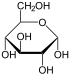
In solutions, the open-chain form of glucose (either “D-” or “L-“) exists in equilibrium with several cyclic isomers, each containing a ring of carbons closed by one oxygen atom. In aqueous solution however, more than 99% of glucose molecules, at any given time, exist as pyranose. The open-chain form is limited to about 0.25% and furanose exists in negligible amounts. The terms “glucose” and “D-glucose” are generally used for these cyclic forms as well. The ring arises from the open-chain form by a nucleophilic addition reaction between the aldehyde group -(C=O)H at C-1 and the hydroxyl group -OH at C-4 or C-5, yielding a hemiacetalgroup -C(OH)H-O-.
The reaction between C-1 and C-5 creates a molecule with a six-membered ring, called pyranose, after the cyclic ether pyran, the simplest molecule with the same carbon-oxygen ring. The (much rarer) reaction between C-1 and C-4 creates a molecule with a five-membered ring, called furanose, after the cyclic ether furan. In either case, each carbon in the ring has one hydrogen and one hydroxyl attached, except for the last carbon (C-4 or C-5) where the hydroxyl is replaced by the remainder of the open molecule (which is -(C(CH2OH)HOH)-H or -(CHOH)-H, respectively).
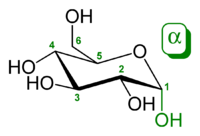
The ring-closing reaction makes carbon C-1 chiral, too, since its four bonds lead to -H, to -OH, to carbon C-2, and to the ring oxygen. These four parts of the molecule may be arranged around C-1 (the anomeric carbon) in two distinct ways, designated by the prefixes “α-” and “β-“. When a glucopyranose molecule is drawn in the Haworth projection, the designation “α-” means that the hydroxyl group attached to C-1 and the -CH2OH group at C-5 lies on opposite sides of the ring’s plane (a trans arrangement), while “β-” means that they are on the same side of the plane (a cis arrangement).
Therefore, the open-chain isomer D-glucose gives rise to four distinct cyclic isomers: α-D-glucopyranose, β-D-glucopyranose, α-D-glucofuranose, and β-D-glucofuranose. These are all chiral.
 α-D– α-D–
Glucopyranose |
 β-D– β-D–
Glucopyranose |
 α-D– α-D–
Glucofuranose |
 β-D– β-D–
Glucofuranose |
The other open-chain isomer L-glucose similarly gives rise to four distinct cyclic forms of L-glucose, each the mirror image of the corresponding D-glucose.
The rings are not planar, but are twisted in three dimensions. The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the “chair” and “boat” conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the “envelope” conformations of cyclopentane.
The glucopyranose forms of glucose predominate in solution, and are the only forms observed in the solid state. They are crystalline colorless solids, highly soluble in water and acetic acid, poorly soluble in methanol and ethanol. They melt at 146 °C (295 °F) (α) and 150 °C (302 °F) (β), and decompose at higher temperatures into carbon and water.
Rotational isomers[edit]
Each glucose isomer is subject to rotational isomerism. Within the cyclic form of glucose, rotation may occur around the O6-C6-C5-O5 torsion angle, termed the ω-angle, to form three staggered rotamer conformations called gauche–gauche (gg), gauche–trans (gt) and trans–gauche (tg). For methyl α-D-glucopyranose at equilibrium the ratio of molecules in each rotamer conformation is reported as 57:38:5 gg:gt:tg.[14] This tendency for the ω-angle to prefer to adopt a gaucheconformation is attributed to the gauche effect.
Physical properties[edit]
Solutions[edit]
All forms of glucose are colorless and easily soluble in water, acetic acid, and several other solvents. They are only sparingly soluble in methanol and ethanol.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation.[15] Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the anomeric effect.[16] Mutarotation is considerably slower at temperatures close to 0 °C (32 °F).
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different -OH group than the one recreated by the opening step (thus switching between pyranose and furanose forms), and/or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
Solid state[edit]
Depending on conditions, three major solid forms of glucose can be crystallised from water solutions: α-glucopyranose, β-glucopyranose, and β-glucopyranose hydrate.[17]
Optical activity[edit]
Whether in water or in the solid form, D-glucose is dextrorotatory, meaning it will rotate the direction of polarized light clockwise. The effect is due to the chirality of the molecules, and indeed the mirror-image isomer, L-glucose, is levorotatory (rotates polarized light counterclockwise) by the same amount. The strength of the effect is different for each of the five tautomers.
Note that the D– prefix does not refer directly to the optical properties of the compound. It indicates that the C-2 chiral center has the same handedness as that of D-glyceraldehyde (which was so labeled because it is dextrorotatory). The fact that D-glucose is dextrorotatory is a combined effect of its four chiral centers, not just of C-2; and indeed some of the other D-aldohexoses are levorotatory.
Computational Models[edit]
Glucose physical and chemical properties have been used to build a computational model of protein-glucose binding-sites.[18]
Production[edit]
Biosynthesis[edit]
In plants and some prokaryotes, glucose is a product of photosynthesis. In animals and fungi, glucose results from the breakdown of glycogen, a process known asglycogenolysis. In plants the breakdown substrate is starch.
In animals, glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate, lactate and glycerol, by a process known asgluconeogenesis.
In some deep-sea bacteria, glucose is produced by chemosynthesis.
Commercial[edit]
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat, cassava, corn huskand sago are all used in various parts of the world. In the United States, corn starch (from maize) is used almost exclusively. Most commercial glucose occurs as a component of invert sugar, a roughly 1:1 mixture of glucose and fructose. In principle, cellulose could be hydrolysed to glucose, but this process is not yet commercially practical.[17] Glucose has approximately 75% the sweetness of sucrose (table sugar).[19]
Sources and absorption[edit]
Most dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or together with another monosaccharide, as in sucrose and lactose.
In the lumen of the duodenum and small intestine, the glucose oligo- and polysaccharides are broken down to monosaccharides by the pancreatic and intestinal glycosidases. Other polysaccharides cannot be processed by the human intestine and require assistance by intestinal flora if they are to be broken down; the most notable exceptions are sucrose (fructose-glucose) and lactose (galactose-glucose). Glucose is then transported across the apical membrane of the enterocytes bySLC5A1 (SGLT1), and later across their basal membrane by SLC2A2 (GLUT2).[20] Some of the glucose is converted to lactic acid by astrocytes, which is then utilized as an energy source by brain cells; some of the glucose is used by intestinal cells and red blood cells, while the rest reaches the liver, adipose tissue andmuscle cells, where it is absorbed and stored as glycogen (under the influence of insulin). Liver cell glycogen can be converted to glucose and returned to the blood when insulin is low or absent; muscle cell glycogen is not returned to the blood because of a lack of enzymes. In fat cells, glucose is used to power reactions that synthesize some fat types and have other purposes. Glycogen is the body’s “glucose energy storage” mechanism, because it is much more “space efficient” and less reactive than glucose itself.
In hypoglycemia management[edit]
Individuals with diabetes or other conditions where hypoglycemia (low blood sugar) may occur often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder).
History[edit]
Glucose was first isolated (from raisins) in 1747 by the German chemist Andreas Marggraf.[21] Because glucose is a basic necessity of many organisms, a correct understanding of its chemical makeup and structure contributed greatly to a general advancement in organic chemistry. This understanding occurred largely as a result of the investigations of Emil Fischer, a German chemist who received the 1902 Nobel Prize in Chemistry for his findings.[22] The synthesis of glucose established the structure of organic material and consequently formed the first definitive validation of Jacobus Henricus van’t Hoff‘s theories of chemical kinetics and the arrangements of chemical bonds in carbon-bearing molecules.[23] Between 1891 and 1894, Fischer established the stereochemical configuration of all the known sugars and correctly predicted the possibleisomers, applying van’t Hoff’s theory of asymmetrical carbon atoms. |


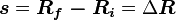
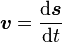 (where ds is an
(where ds is an